Duxin Group

The team is specifically interested in understanding how cytotoxic DNA lesions known as DNA-protein crosslinks (DPCs) are tolerated by cells and cleared from the genome.
These lesions are generated by mainstream chemotherapeutics (e.g. topoisomerase poisons) but are also the common byproduct of cellular metabolism (e.g. formaldehyde).
DPCs are common DNA lesions generated by a wide variety of chemotherapeutic and crosslinking agents. In fact, many of the mainstreams chemotherapeutic drugs used in the clinic cause DPCs. Failure to repair DPCs interfere with DNA metabolism processes inducing DNA damage and cell death (principle of how chemotherapeutics kill cancer cells). If left unrepaired, DPCs can also threaten genomic integrity of healthy cells causing accelerated ageing and cancer in humans (the double edge sword of chemotherapy).
“Understanding DPC repair is critical for human health, as many chemotherapeutic drugs used in the clinic exert their toxicity by generating DPCs and we know very little about the processes involved in clearing these lesions from the genome,” says Associate Professor and Group Leader Julien Duxin.
To this end, the Duxin group takes advantage of a protein extract system derived from eggs from African clawed frogs (Xenopus laevis). These protein extracts have the remarkable capacity to reiterate processes of DNA replication and DNA repair in a cell-free environment, providing a unique opportunity to delineate the molecular mechanisms underlying complex DNA transactions.
“Using frog egg extracts we are able to recapitulate DPC-repair in a test tube. This allows us to manipulate this process and interrogate which are the main players orchestrating this reaction,“ says Julien Duxin.
Novel role of Polymerase kappa in DNA damage bypass and tolerance
By combining CRISPR base editor screening technology in human cells with translesion synthesis analysis of defined DNA lesions in Xenopus egg extracts the Duxin group unraveled the functions and regulations of Polymerase kappa during DNA lesion bypass.
Strikingly, they identified a novel non-catalytic function of Polymerase kappa in stimulating Polymerase Zeta-mediated bypass of different DNA lesions such as DPCs and abasic sites.
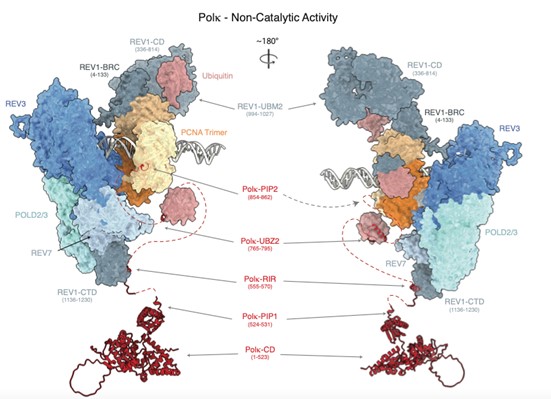
Composite molecular model of the and non-catalytic function of Polymerase Kappa during translesion DNA synthesis. Models were generated by combining structure predictions from AlphaPulldown (Yu et al., 2023) / AlphaFold2 (Jumper et al., 2021). The model was generated in collaboration with Alberto Carli and Thomas Miller (UCPH).
New Role for ubiquitin ligase RFWD3
Using Xenopus egg extracts the Duxin group identified a new role for the ubiquitin ligase RFWD3. They found that RFWD3 promotes ubiquitylation of PCNA and proteins on single-stranded DNA to stimulate gap filling DNA synthesis across different polymerase-blocking DNA lesions including DNA-protein and DNA-interstrand crosslinks. This provides a molecular understanding of RFWD3’s essential function in safeguarding the genome.
Find the study "The ubiquitin ligase RFWD3 is required for translesion DNA synthesis" here.
Mechanisms of replication-coupled DPC proteolysis
A new study from the Duxin group presents two mechanisms of replication-coupled DPC proteolysis and provide the molecular links that couple these processes to DNA replication. By employing different DPC proteases, cells possess a versatile system that can cope with a wide diversity of DPCs.
Our ongoing lab projects center around unraveling the intricate molecular mechanisms involved in repairing DNA-protein crosslinks (DPCs) and bypassing of DNA lesions during the process of DNA replication:
- Delineate the repair mechanisms underlying TOP1-and TOP2-DPC (also known as TOP1cc/TOP2cc) induced by mainstream topoisomerase poisons/inhibitors.
To this end we recapitulate the repair reactions in egg extracts and study the roles and functions of the key players orchestrating repair. - Understand the impact of formaldehyde induced chromatin lesions on DNA replication. While formaldehyde is thought to be the main “endogenous” DNA damaging agents, little is known about the nature of the lesions that formaldehyde induces and the enzymes and repair pathway counteracting these lesions. Using a unique approach using Xenopus egg extracts we are trying to shed light on these questions.
- Delineate the Mechanisms and molecular triggers of DNA damage bypass by translesion DNA synthesis and template switching. How are translesion synthesis and template switching mechanisms regulated? This is a long-term project in the lab aimed at understanding this fundamental question.
In the lab, we combine biochemical work in Xenopus egg extracts, CRISPR/Cas9 screening technologies in human cells, mass spectrometry, and structural work (Cryo-EM) to delineate molecular mechanisms underlying DNA repair and replication mechanisms.
For example, we have developed mass spectrometry approaches to isolate protein complexes from plasmids containing defined DNA lesions in egg extracts:
- Mol Cell. 2019 Feb 7;73(3):574-588.e7. doi: 10.1016/j.molcel.2018.11.024.
(in collaboration with Markus Raschle and Matthias Mann)
Developed a novel mass spectrometry approach to identify de novo ubiquitylation events in egg extracts:
- Nat Commun. 2023 Dec 14;14(1):8293. doi: 10.1038/s41467-023-43873-0. PMID: 38097601racts
(in collaboration with Michael Lund Nielsen, UCPH)
Performed CRISPR/cas9 editor screens to point at the critical interactions related to DNA damage tolerance and bypass:
- bioRxiv 2023.11.06.565773; doi: https://doi.org/10.1101/2023.11.06.565773
(in collaboration with Jakob Nilsson, UCPH) - Using structural approaches (via Cryo-EM) to investigate native protein complexes isolated from Xenopus egg extracts (in collaboration with Tom Miller, UCPH)
September 2023
Two Former CPR Post-Docs Awarded Prestigious ERC Starting Grants, read story here.
Meet the Duxin Lab
Our philosophy is to have fun while doing thorough science!























