Kummer Group
The Kummer group has a deep interest in understanding the biology of human mitochondria with a specific focus on how these important organelles maintain their DNA and how they produce functional RNA species. Strikingly, the mitochondrial replication system shows similarities to viral replication proteins and we more recently expanded our research to investigate how important human pathogenic viruses with currently limited options for treatment propagate their DNA in the human host.
Kummer Group are hiring a Postdoc in Cryo-EM of Viral Replication Systems. Read more through this link. Application deadline: January 19, 2025.
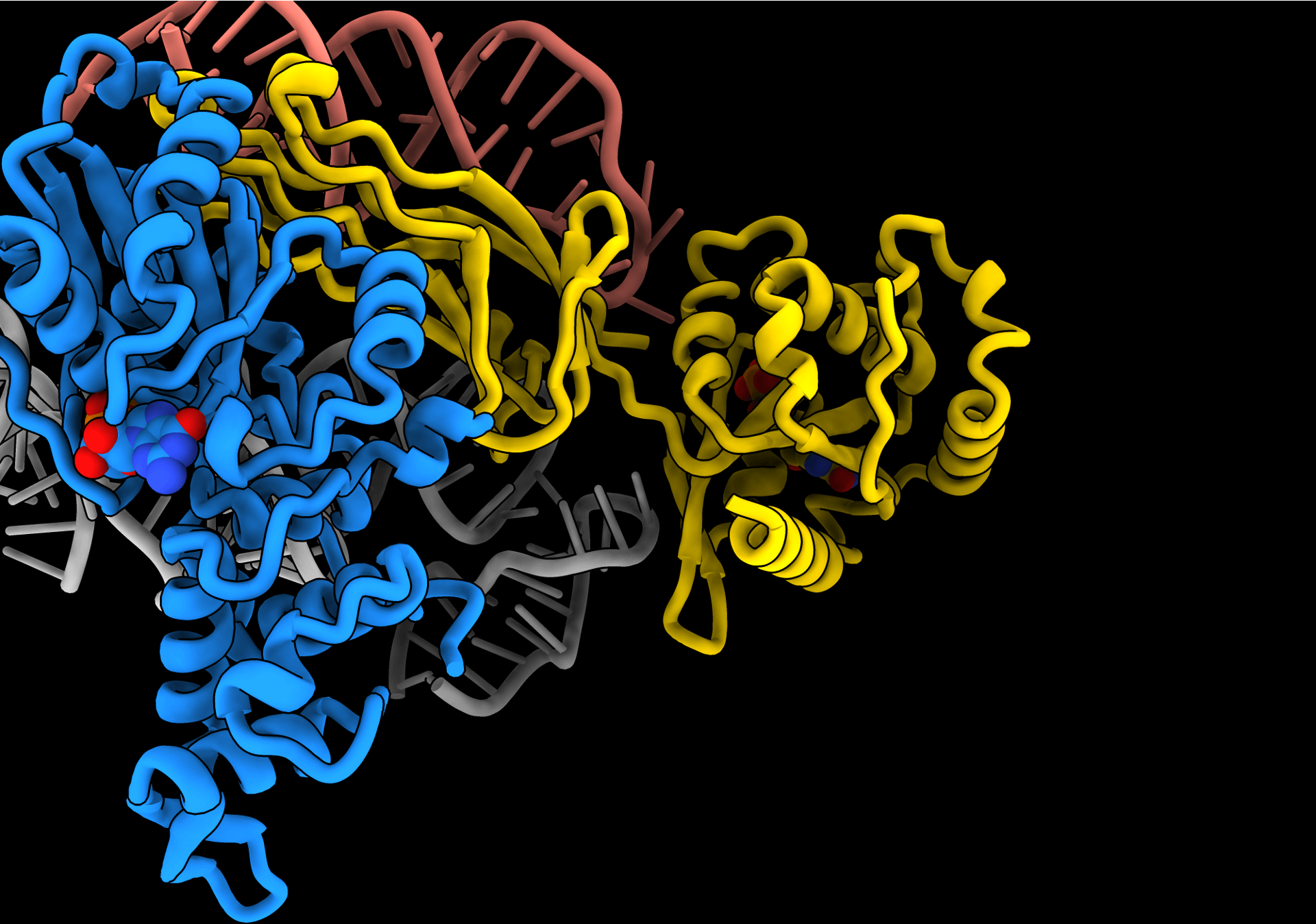
We combine structural approaches with functional biochemistry and cell biology to investigate essential cellular processes in molecular detail. Our structural analysis is primarily based on single particle cryo-EM.
Our research focusses on two main areas:
- We aim to gain fundamental mechanistic insights into protein factors that mediate mitochondrial DNA maintenance and RNA maturation with the hope to shed light on the molecular triggers of mitochondrial disorders that are frequently caused by mutations in these proteins.
- More recently, we started to investigate how members of the family of human herpesviruses replicate their DNA during active infection. Prominent examples of include Herpes Simplex Virus 1 and 2, Epstein Barr Virus, Cytomegalovirus and Kaposi Sarcoma-associated Virus that cause a variety of pathologies ranging from cold sores to cancer. Since decades, treatment of herpes infections has been limited to the use of nucleoside analogs to block viral replication and new therapies are needed. Our goal is to generate structural and functional insights into viral replication to enable the development of alternative drug candidates and treatment options in the future.
As independent PI:
Biogenesis of the mitochondrial ribosome
Ribosomes are essential as they are the only protein-producing entities in all organisms. They are complex particles assembled from over 80 RNA and protein components and defects in ribosome assembly severely reduce the capacity of our cells to make proteins. In this study, we visualized how the biogenesis factors GTPBP10 and GTPBP7 place RNA component H89 during assembly of the mitochondrial ribosome to form a functional catalytic peptidyl transferase center.
-
Nguyen, T.G., Ritter, C., Kummer, E. (2023) Structural insights into the role of GTPBP10 in the RNA maturation of the mitoribosome, Nature Communications.
Prior to independent PI:
Protein synthesis in mammalian mitochondria
Cryo-EM studies unravelling specific structural and functional adaptions in the process of protein production that mammalian mitochondria have acquired during evolution. Among these studies is the first reconstituted mitochondrial translation complex.
- Kummer, E., Schubert, K.N., Schoenhut, T., Scaiola, A., Ban, N. (2021) Structural basis of translation termination, rescue, and recycling in mammalian mitochondria, Mol Cell.
- Kummer, E., and Ban, N. (2020) Structural insights into mammalian mitochondrial translation elongation catalyzed by mtEFG1, EMBO J.
- Kummer, E., Leibundgut, M., Rackham, O., Lee, R. G., Boehringer, D., Filipovska, A., Ban, N. (2018) Unique features of mammalian mitochondrial translation initiation revealed by cryo-EM, Nature.
- Kummer, E. and Ban, N. (2021) Mechanisms and regulation of protein synthesis in mitochondria, Nature Reviews Molecular Cell Biology,
Sigma adaption in mycobacteria
In a collaboration project, I have obtained the cryo-EM structure of a mycobacterial transcription initiation complex that is critical for the pathogen to adapt to cellular stress. We found that the transcription activator PafBC employs an unusual mechanism to regulate gene expression by altering the promoter specificity of bacterial sigma factors – a phenomenon that we have termed sigma adaption.
- Müller, A.U.*, Kummer, E.*, Schilling, C.M., Ban, N., Weber-Ban, E. (2021) Transcriptional control of mycobacterial DNA damage response by sigma adaptation, Science Advances.
Reversion of protein aggregation by concerted action of molecular chaperones
Unravelled a novel principle of how two chaperone systems disentangle aggregating, misfolded proteins inside the cell. Showed that direct interaction of the chaperones tunes their activity in space and time in order to adequately respond to cellular needs and prevent detrimental off-target effects. Bacteria and fungi thereby manage to sustain a healthy proteome at a relatively low energetic expense.
- Carroni, M., Kummer, E., Oguchi, Y., Wendler, P., Clare, D. K., Sinning, I., Kopp, J., Mogk, A., Bukau, B., Saibil, H. (2014) Head-to-tail interactions of the coiled-coil domains regulate ClpB cooperation with Hsp70 in protein disaggregation, Elife,
- Seyffer, F.*, Kummer, E.*, Oguchi, Y., Winkler, J., Kumar, M., Zahn, R., Sourjik, V., Bukau, B., Mogk, A. (2012) Hsp70 proteins bind Hsp100 regulatory M domains to activate AAA+ disaggregase at aggregate surfaces, Nat Struct Mol Biol,
- Oguchi, Y.*, Kummer, E.*, Seyffer, F., Berynskyy, M., Anstett, B., Zahn, R., Wade, R. C., Mogk, A., Bukau, B. (2012) A tightly regulated molecular toggle controls AAA+ disaggregase, Nat Struct Mol Biol,
We aim to obtain a molecular understanding of the operating principles of nucleic-acid binding and processing proteins using structural and biochemical approaches. Our areas of interest include:
- The replication machinery of mammalian mitochondrial DNA
- Protein complexes involved in mitochondrial gene expression including RNA maturation and translation
- Viral replication systems with a specific focus on members of Herpesviridae
Our main interest is to understand the structure and function of proteins and protein – nucleic acid complexes. To this end, we obtain our proteins of interest by recombinant expression in bacterial, insect and mammalian cells. Structural studies are primarily carried out using single particle transmission electron microcopy, for which we have access to all relevant equipment for negative stain and cryogenic samples at the Core Facility for Integrated Microscopy including vitrification devices, as well as Glacios and Krios TEM. To analyze integrity, oligomeric state, and substrate interactions of our proteins we combine functional biochemical assays and biophysical approaches such as mass photometry via equipment at the Protein Production and Characterization Platform at the Center for Protein Research.




